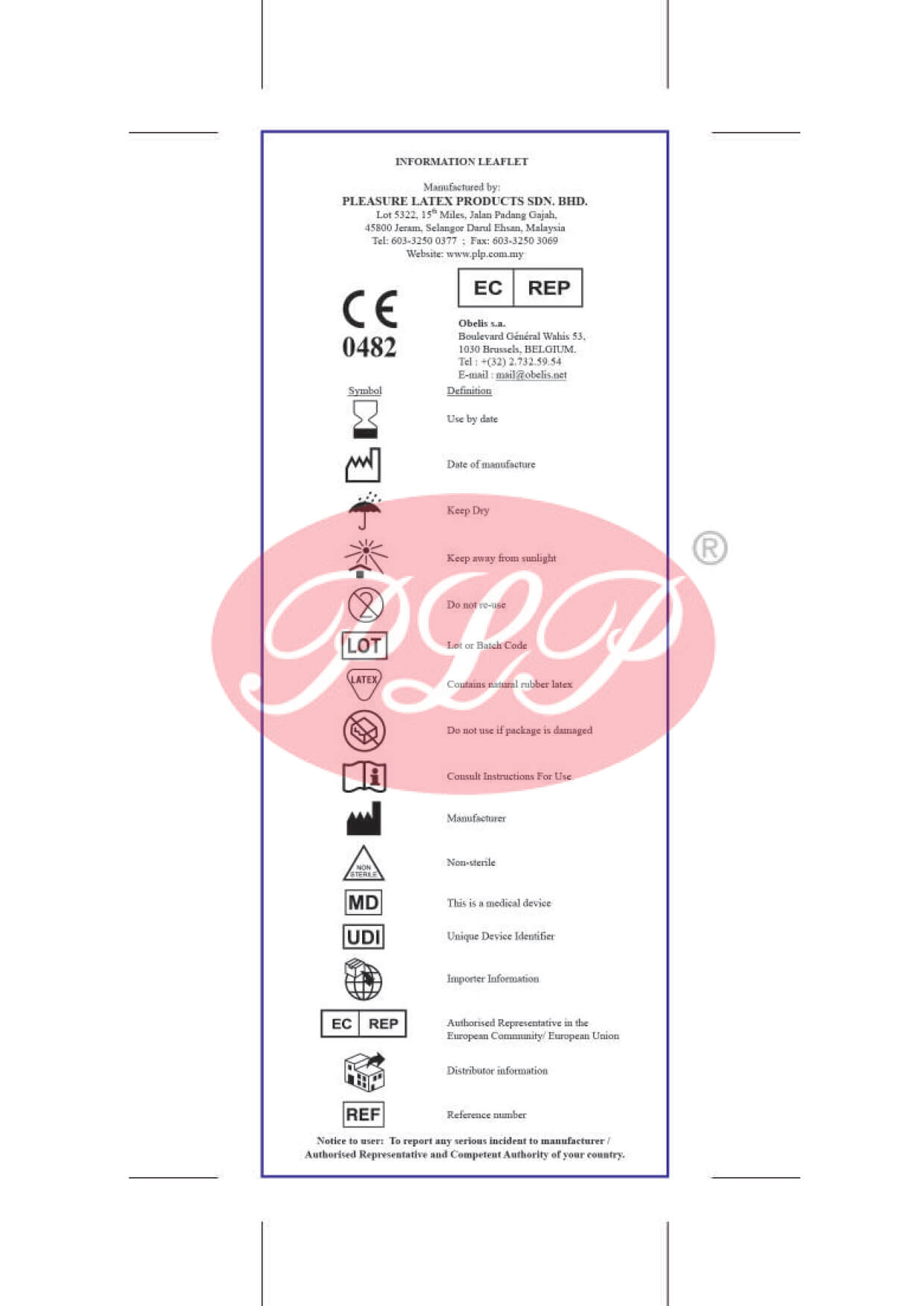
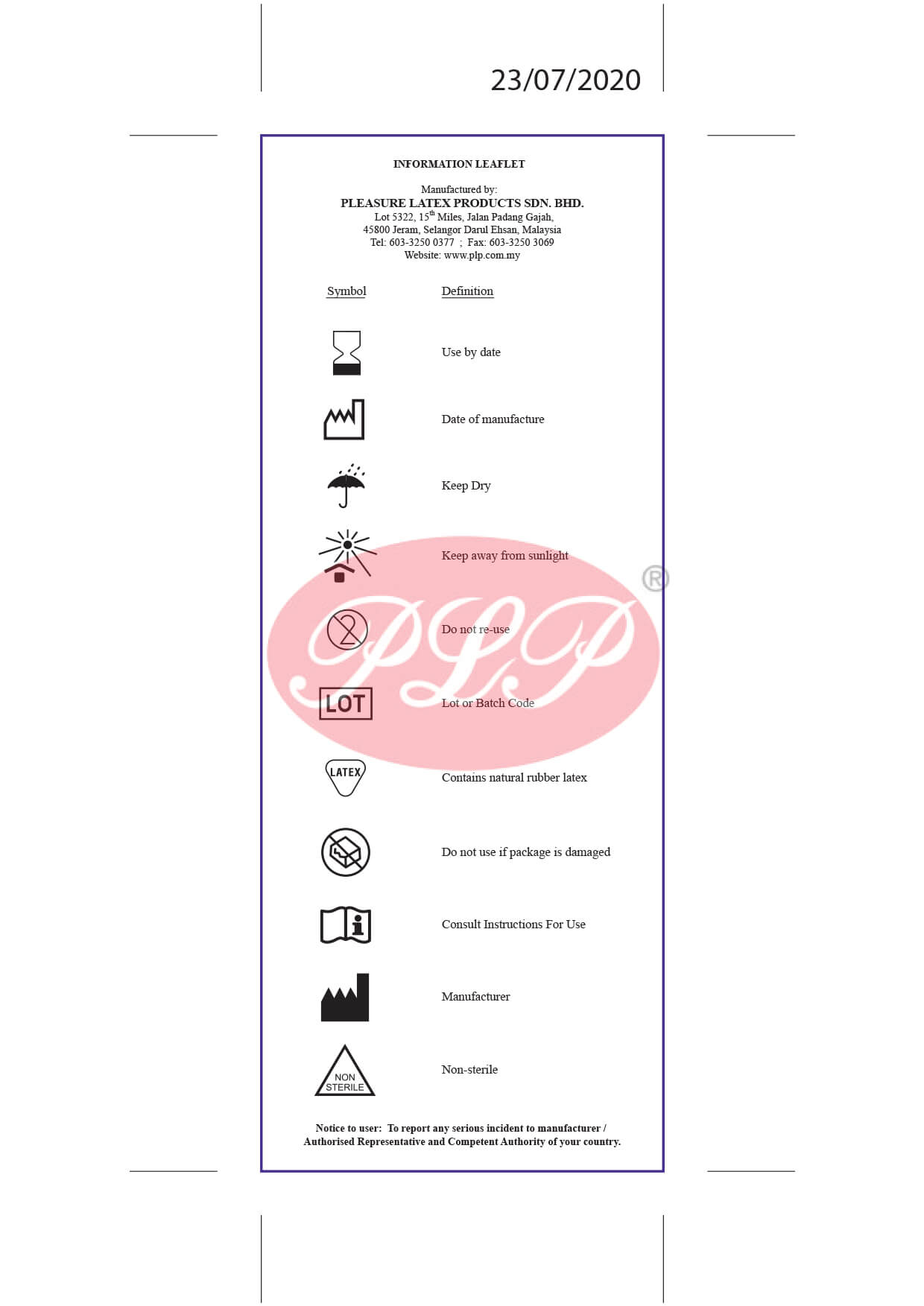
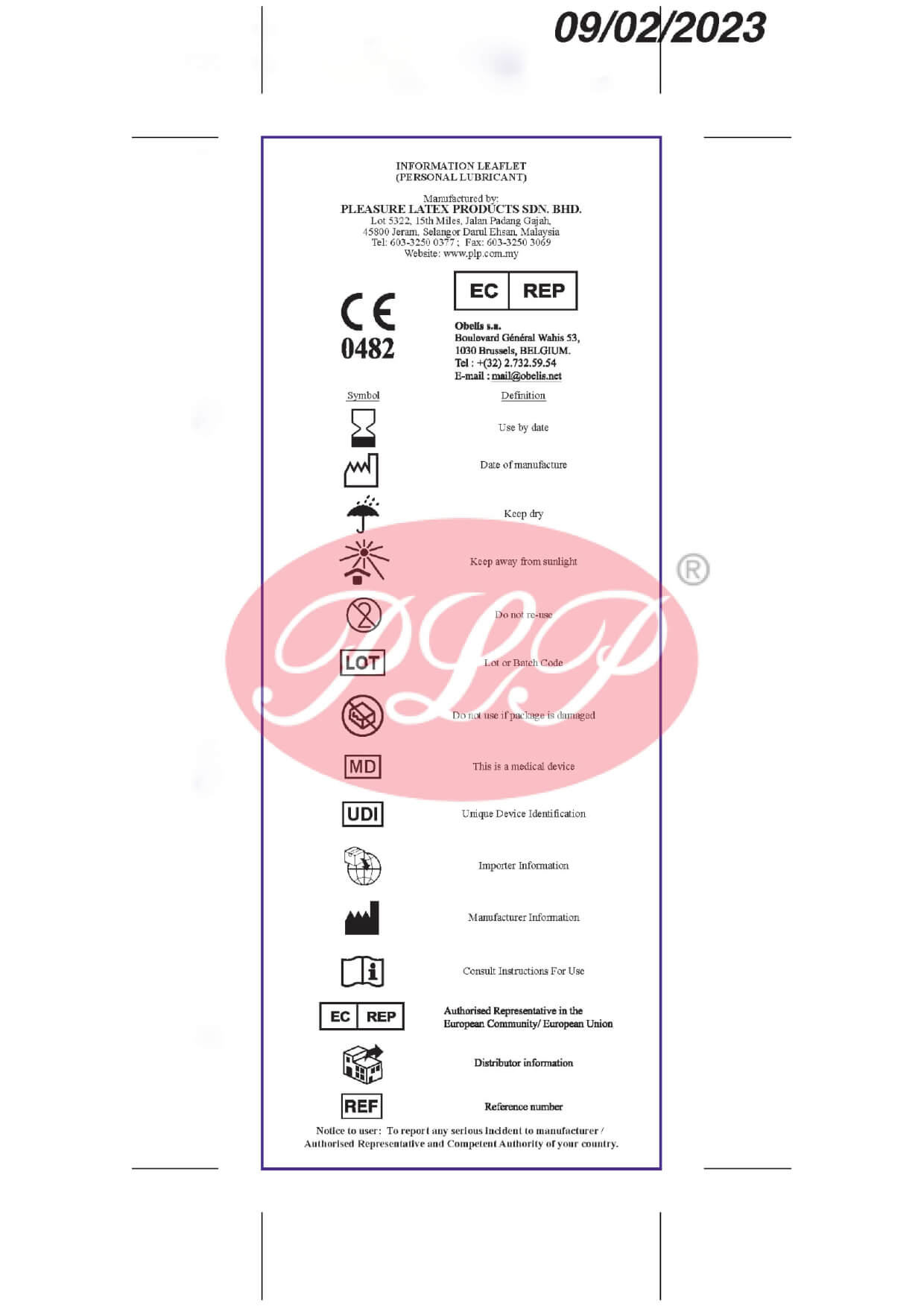
Quality Policy
Policy Statement
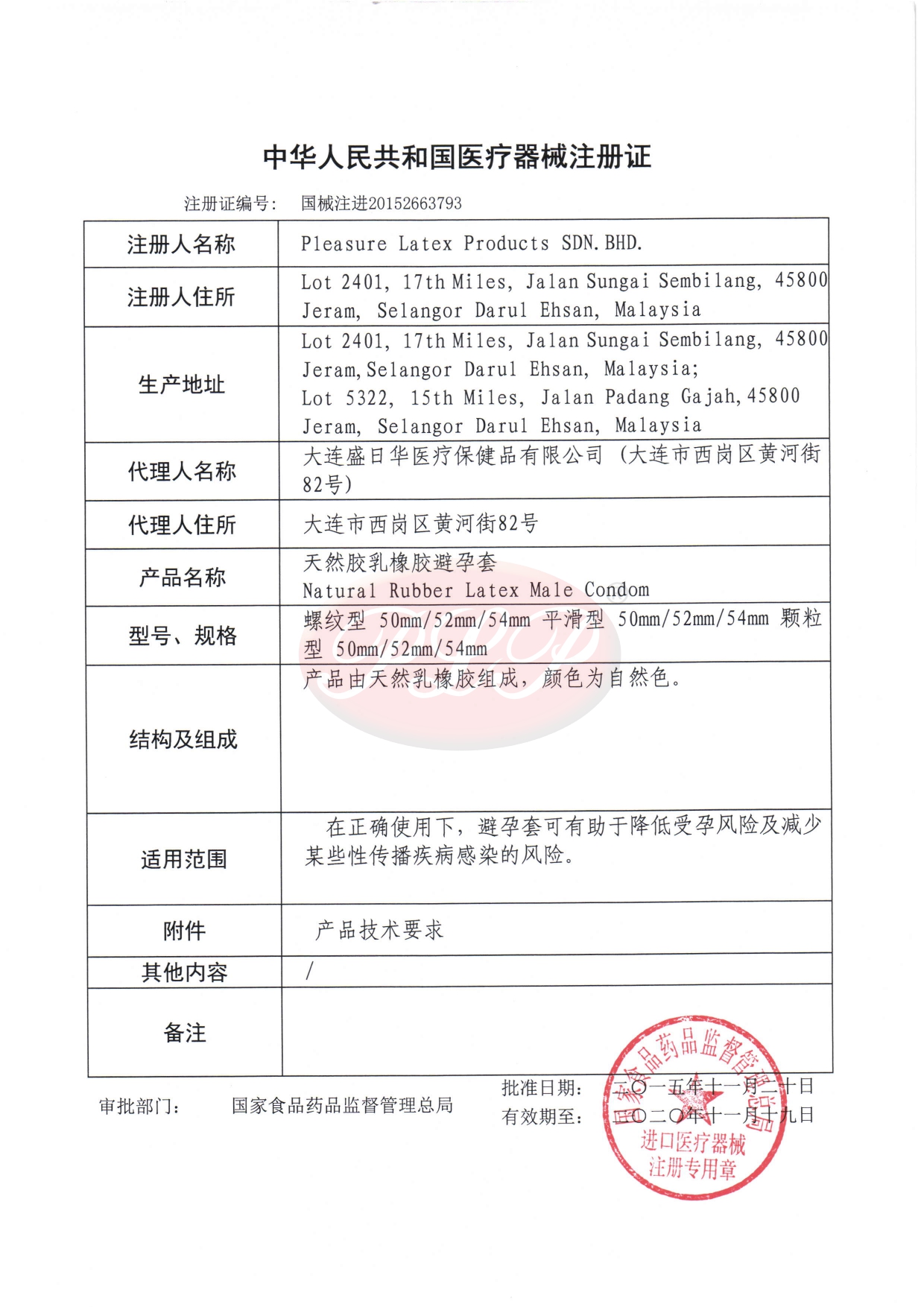
We, PLEASURE LATEX PRODUCTS SDN. BHD., manufacturer of natural rubber latex male condom and related products is committed to our customers by providing quality products with prompt delivery through good communications.
Missions
In tandem with our quality commitment, it is our policy to:-
Pursue towards meeting customer requirements with good professional practice in compliance with relevant statutory, regulatory and accreditation requirements.
Liase closely the quality management system, which include the quality manual and procedures to ensure that is communicated, understood, implemented and maintained within the organisation.
Provide training to familiarize and continually improve the effectiveness of the quality management system and quality policy.
Strive for continual improvement in practical new technologies and process performance towards meeting quality objectives which are to be reviewed for continuing suitability.
Build customer satisfaction in fulfilling customer contractual requirements for quality.
Objectives
Our objectives towards the Quality policy shall be established, measured and revised annually at management review meeting for continuing suitability.
Goals
To achieve the fundamental goals of this Quality Policy, all manufacturing activities in the organisation shall comply with the following quality system requirements:-
- ISO 9001
- ISO 13485 and its regulatory variants
- US FDA's Quality System Requirement (QSR)
Certificates
Product Mark
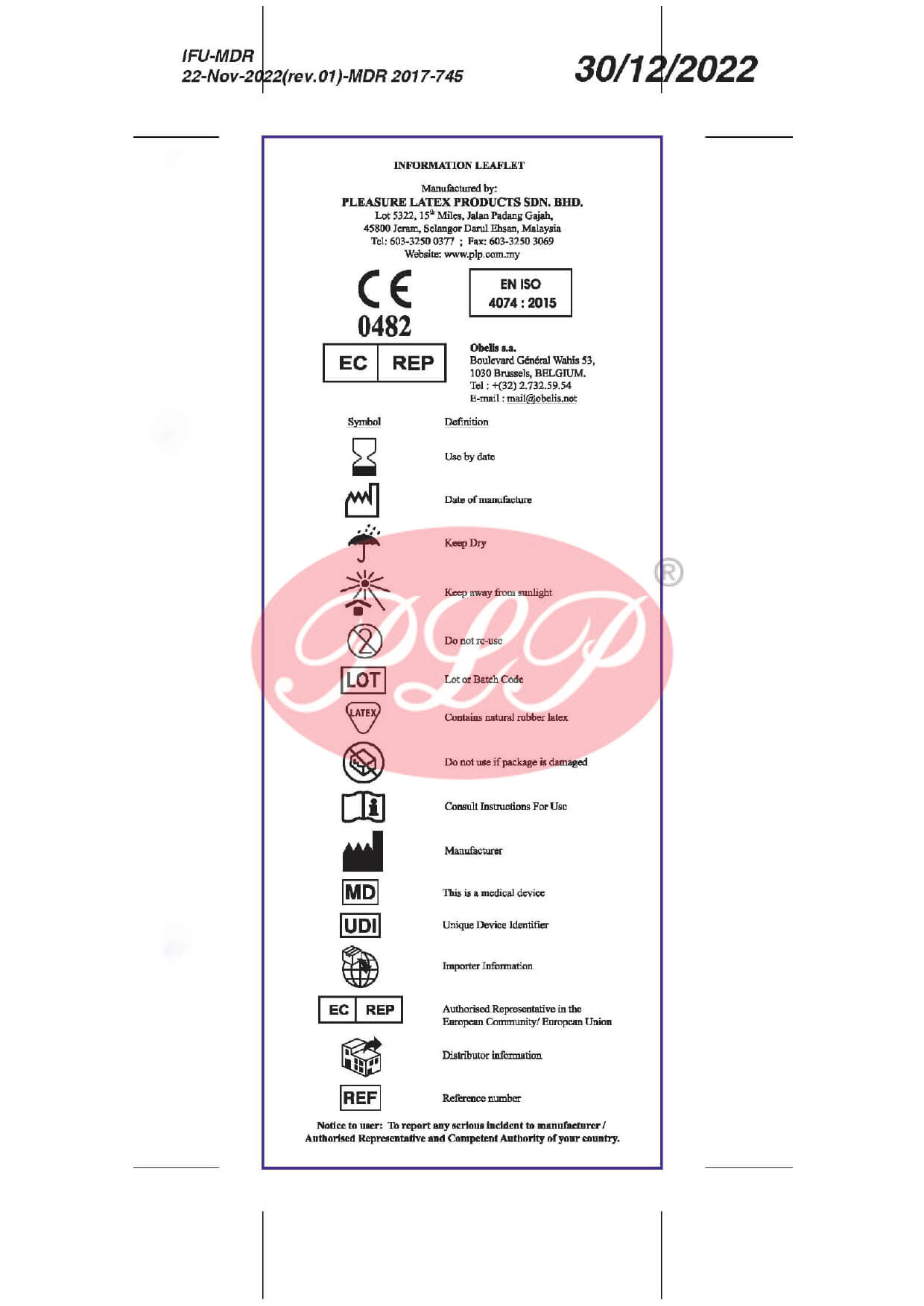
- CE Mark
Europe
- SIRIM Mark
Malaysia
Registered With
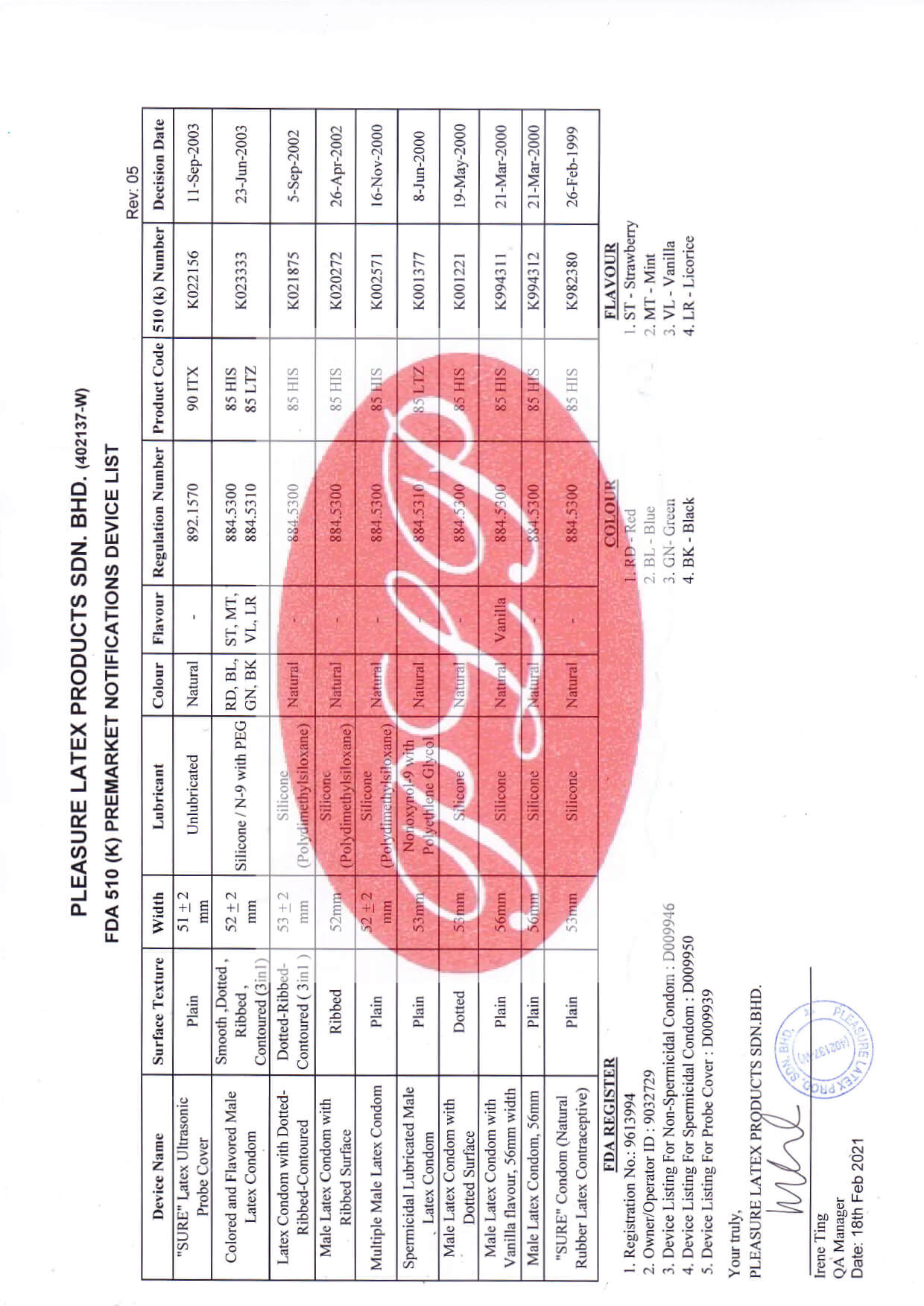
- FDA 510(k)
USA - MDA
Malaysia - CFDA
China
Testing Standard
- ISO 4074
Well-Recognised International Standard - WHO
World Health Organisation Tender Specification - ASTM
United State ASTM Standard - GB 7544
China National Standard